Prevention of Peanut Allergy
NIAID Guidelines and Background

Recent studies, including the Learning Early About Peanut (LEAP) study, demonstrate that early introduction of peanuts in children considered at high risk of developing peanut allergy can decrease peanut allergy. Based on the LEAP study, the NIAID Addendum Guidelines were released in 2017 and outline strategies for the early introduction of peanuts into the diets of all children in the United States [3]. They currently recommend the introduction of developmentally appropriate forms of peanut (with a goal of about 2grams of peanut protein, 3x a week) and outline timing, developmentally appropriate forms of peanut, how to assess risk, etc [3].
The American Academy of Pediatrics (AAP), the US Department of Agriculture (USDA), and the US Department of Health and Human Services (HHS) have released guidance documents that support the above recommendations. The NIAID guidelines suggest screening in high-risk children, those with severe eczema and/or an egg allergy, and no screening is necessary for children with no risk factors. Screening is also unnecessary for positive family history of allergy, and mild and moderate eczema.
The AAP released a 2019 Clinical Report that endorsed the NIAID Addendum Guidelines. This report includes the mention of potential issues with the screening recommendation. In this report, they stated that “It is hoped that the screening process for the infants at highest risk (specific IgE measurement, skin-prick test, and oral food-challenge test) will not be a deterrent or generate “screening creep” for infants not in the high-risk category.” These guidelines may be difficult to follow in communities without access to the medical care needed for implementation. Also relevant is the statement, “The guidance in this report does not indicate an exclusive course of treatment or serve as a standard of medical care. Variations, taking into account individual circumstances, may be appropriate” (6).
Main Takeaways
- Early introduction of peanuts in high-risk children, as recommended by the NIAID Addendum Guidelines, has been shown to decrease the risk of developing peanut allergy.
- The AAP, USDA, and HHS endorse the NIAID recommendations, providing clear screening criteria for high-risk children (severe eczema and/or egg allergy). Children with no risk factors; children with mild or moderate eczema; and children with a positive family history of allergy do not require screening.
- The AAP’s 2019 Clinical Report supported the NIAID Addendum Guidelines, but noted potential screening challenges. They hoped that high-risk infant screening wouldn’t affect low-risk infants. Implementing the guidelines in communities with limited medical access could be difficult. The report emphasized flexibility in treatment, tailored to individual circumstances, rather than a strict standard of care.
NIAID Addendum Guideline Summary
| Infant Criteria | Recommendations | Earliest Age of Peanut Introduction |
|---|---|---|
| Severe eczema, egg allergy, or both | Strongly consider evaluation with peanut-specific IgE and/or skin prick test and, if necessary, an oral food challenge. Based on test results, introduce peanut-containing foods. | 4 to 6 months |
| Mild to moderate eczema | Introduce peanut-containing foods. | Around 6 months |
| No eczema or any food allergy | Introduce peanut-containing foods. | Age appropriate and in accordance with family preferences and cultural practices |
High Risk Infants
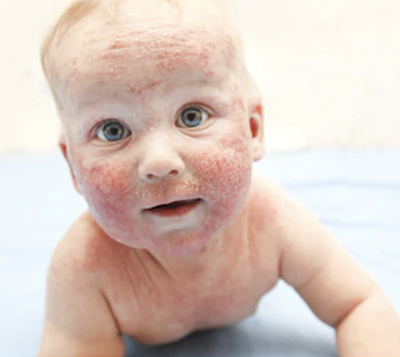
Infants considered high-risk for peanut allergy are those with severe eczema, egg allergy, and/or any other known IgE-mediated food allergy. The NIAID defines severe eczema as “Persistent or frequently recurring eczema with typical morphology and distribution assessed as severe by a healthcare provider and requiring frequent need for prescription-strength topical corticosteroids, calcineurin inhibitors, or other anti-inflammatory agents despite appropriate use of emollients (3).” Hospital systems may have varied approaches to implementing the NIAID guidelines.
According to the guidelines, referring them to a pediatric allergist for evaluation and possible skin testing is recommended. You can also send a blood test for peanut-specific IgE, and if that is positive, order a referral for a pediatric allergist.
If the peanut-specific IgE is negative, you can advise the family to introduce peanuts at home when the infant is 4-6 months of age or ready to try solid food. You can provide them with a copy of the handout entitled “Introducing Peanut Products to Your Baby.”
Feel free to view the sample approach by Lurie Children’s Hospital on implementing the NIAID guidelines within their system.
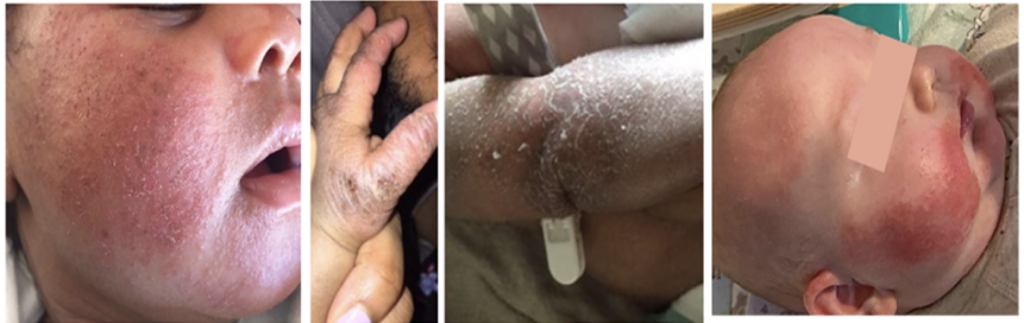
Moderate Risk Infants

These infants are those with mild to moderate eczema or another food allergy. For these infants, you can recommend the families introduce peanuts at home, starting at 6 months of age, and follow the family’s preferences and cultural practices. Consider referring the family to an allergist to manage the known food allergy, but do not delay peanut introduction. Provide them with a copy of the handout entitled “Introducing Peanut Products to Your Baby”.
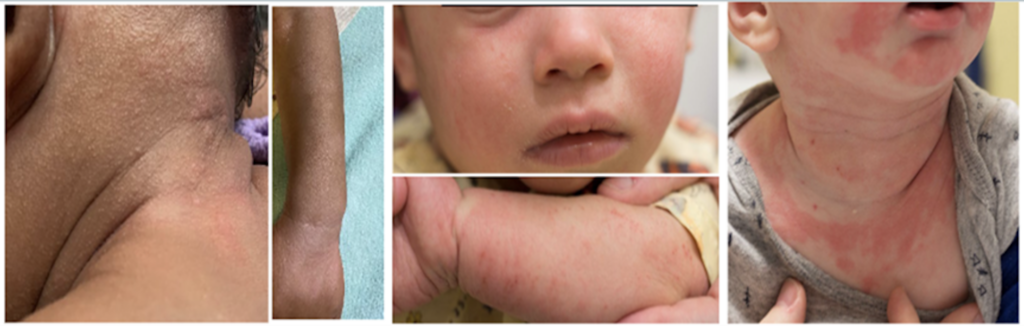
Low Risk Infants

Infants who have no eczema or food allergy are considered low risk. For these infants, recommend families to introduce peanuts at home according to their individual preferences and cultural practices. Provide them with a copy of the handout entitled “Introducing Peanut Products to Your Baby”.
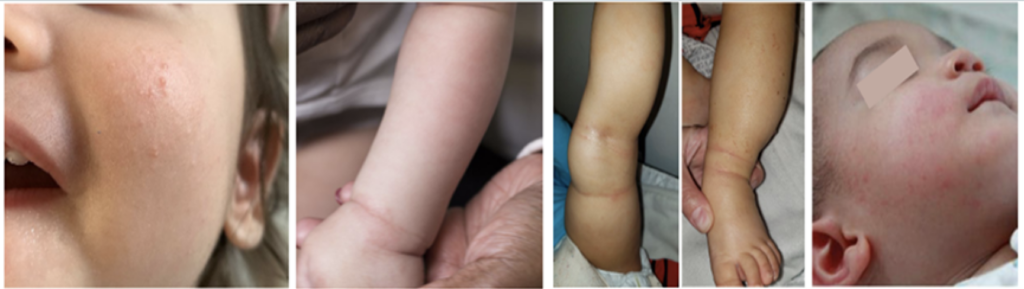
Main Takeaways
- Severe eczema, egg allergy, or both:
- Goal is to introduce peanut-containing foods at 4 to 6 months.
- Consider screening (allergy referral or sending peanut-specific IgE).
- If screening causes delays, consider a supervised feeding.
- Mild to moderate eczema:
- Introduce peanut-containing foods at around 6 months.
- There is no need for screening.
- No eczema or any food allergy:
- Introduce peanut-containing foods at an age-appropriate time, per family preferences and cultural practices.
- There is no need for screening.
Alternatives to NIAID Approach
The American Academy of Allergy, Asthma, and Immunology, the American College of Asthma, Allergy, and Immunology, and the Canadian Society of Allergy and Clinical Immunology support the recent publication- A Consensus Approach to the Primary Prevention of Food Allergy Through Nutrition that de-emphasizes the need for screening that is suggested in the NIAID Addendum Guidelines.
This consensus document recommends that all infants, irrespective of relative risk, introduce peanut-containing foods and egg at 4-6 months when the infant is developmentally ready for complementary foods. Also highlighted is the importance of shared decision-making as families consider the introduction (4).
Currently, the United States is the only country with a screening step. In countries including Australia, Israel, and the United Kingdom without similar screening steps that recommend peanut introduction, reactions are rare. Specifically, the British Society of Allergy and Clinical Immunology (BSACI) guidelines state– “The benefits of allergy testing in higher-risk babies before introducing egg or peanut needs to be balanced against the risk this could cause a delay (due to lack of available testing) and increase the risk of food allergy”(5). Even in infants who already have food allergies, including those with severe eczema, “The risk of a severe reaction (anaphylaxis) is low (1-2 per 1000) in these babies” (5).
Since the 2017 release of the NIAID guidelines and the 2019 release of the American Academy of Pediatrics Clinical Report, Keet and colleagues have published on the use of Arah2, and have reported that it is a more sensitive and specific screening tool for clinically relevant peanut allergy as compared to peanut-specific IgE or peanut skin prick test in high-risk infants (6).
Main Takeaways
- The American Academy of Allergy, Asthma, and Immunology, the American College of Asthma, Allergy, and Immunology, and the Canadian Society of Allergy and Clinical Immunology support a recent consensus approach to food allergy prevention through nutrition.
- The consensus recommends introducing peanut-containing foods and egg at 4-6 months, regardless of relative risk and when the infant is developmentally ready.
- Unlike the United States, other countries like Australia, Israel, and the UK, which do not have similar screening steps, have rare reactions to early peanut introduction.
- Even in infants with existing food allergies or severe eczema, the risk of severe reactions is low.
Timing Matters
Whether you adhere to the NIAID Addendum Guidelines or the Consensus Approach, it is important to target peanut introduction between 4 to 6 months of age.
In the recent publication Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy, Keet and colleagues demonstrated that in kids with severe eczema, the chance of peanut allergy significantly increases with time. While less than 20% of children under 6 months had a positive oral food challenge to peanuts, about 50% of children over 8 months had a positive oral food challenge to peanuts (7). These findings reinforce the importance of not delaying the introduction of peanuts.
You can start talking about peanut introduction with families even in the newborn period and help families anticipate the introduction of peanut-containing foods, as well as other allergens, as early as 4-6 months of life, especially in high-risk infants.
Main Takeaways
- Regardless of the chosen guideline (NIAID or Consensus Approach), introducing peanuts between 4 to 6 months is crucial.
- Severe eczema and age are significant risk factors for peanut allergy in infants; delaying introduction increases the risk.
- Begin discussions about peanut introduction with families early, even in the newborn period, and help them prepare for introducing peanuts and other allergens at 4-6 months, especially for high-risk infants.
Resources for Parents for the Early Introduction of Peanut:
Videos for Parents for Peanut Introduction as per Addendum Guidelines
Test your knowledge
References
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015 Feb 26;372:803-13.
- Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, Brough H, Marrs T, Radulovic S, Craven J, Flohr C. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016 May 5;374:1733-43.
- National Institute for Allergy and Infectious Disease. Addendum Guidelines for the Prevention of Peanut Allergy in the United States.; 2017. doi:10.1097/01.JAA.0000512231.15808.66
- Fleischer DM;Chan ES;Venter C;Spergel JM; Abrams EM;Stukus D;Groetch M;Shaker M;Greenhawt M; “A Consensus Approach to the Primary Prevention of Food Allergy Through Nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology.” The Journal of Allergy and Clinical Immunology. In Practice, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/33250376/.
- Preventing food allergy in your baby. BSACI. https://www.bsaci.org/wp-content/uploads/2020/02/pdf_Infant-feeding-and-allergy-prevention-PARENTS-FINAL-booklet.pdf. Published February 20, 2020. Accessed October 11, 2022.
- Keet C, Plesa M, Szelag D, et al. Ara h 2-specific IgE is superior to whole peanut extract-based serology or skin prick test for diagnosis of peanut allergy in infancy. J Allergy Clin Immunol. 2021;147(3):977-983.e2. doi:10.1016/j.jaci.2020.11.034
- Keet C, Pistiner M, Plesa M, Szelag D, Shreffler W, Wood R, Dunlop J, Peng R, Dantzer J, Togias A. Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy. Journal of Allergy and Clinical Immunology. 2021.
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov.





